This month, you can participate in the CFIA and Health Canada consultation on food labelling, learn about the recent changes to pre-packed food labels, read about the new severe allergy policy at Queen’s University, and find out about a ground-breaking new U.S. report promoting food allergy safety.
Advocacy
You’re invited: CFIA and Health Canada consultation on food labelling
The Canadian Food Inspection Agency (CFIA) and Health Canada want to hear from Canadians on their proposals regarding modernizing food labelling.
They are holding in-person discussions to get your perspective on:
- Labelling information that is important to you
- Proposed improvements to food label information and legibility, and;
- Enhancements to the ways consumers get information on label claims or statements
These sessions are a great opportunity to learn about new labelling changes firsthand and to provide input on these changes. This is your opportunity to help advocate for changes such as better guidance on precautionary statements like “May Contain” and having defined criteria for “allergen-free” statements on pre-packaged food.
In-person consumer sessions dates:
| Regions | Date |
| Atlantic region | Jan. 30 – Feb. 3rd |
| Central region* | Feb. 13 – Feb. 16th |
| Western region | Feb. 14 – Mar. 3rd |
| *Ontario sessions have passed, upcoming sessions are in Quebec only. | |
Space is limited, so register early. Click here to register for an upcoming consumer session.
If you weren’t able to attend the Ontario sessions, or cannot attend any upcoming sessions, you can have your say through their online questionnaire that is open until February 28, 2017.
Food Allergy Canada participated in both the consumer and industry sessions to advocate for better labelling for you and all Canadians with food allergies. Join us and help to make our collective voice heard!
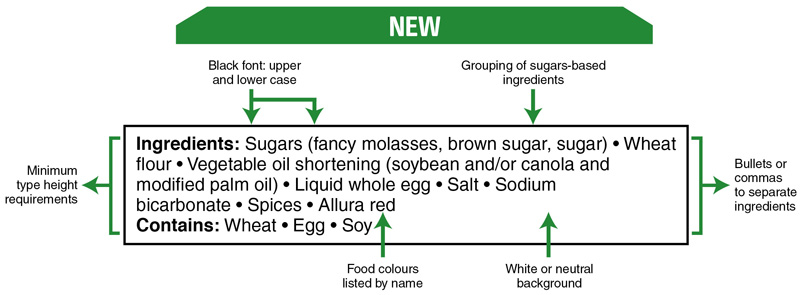
Recent changes to pre-packaged food labels
In December, Health Canada announced changes to pre-packaged food labels that will make it easier for consumers to find, read, and understand the list of ingredients and priority allergens.
Key changes to the list of ingredients include:
- Improved legibility for the list of ingredients and allergen information.
- Grouping of sugars-based ingredients under the common name “sugars”.
- Declaration of food colours by their individual common names.
Food Allergy Canada provided feedback during Health Canada’s consultation period last year and we are pleased to see that many of our suggestions have been incorporated into this new labelling requirement.
Note that manufacturers have a five-year transition period to make these changes.
As always, read ingredient labels with care. For more information, please visit the Health Canada page describing the new amendments.
Here’s a visual of the changes:
Queen’s University Adopts Severe Allergy Policy
Queen’s University has recently launched a new policy to ensure continuous improvement of their campus-wide resources and services to accommodate students with severe allergies.
The policy includes allergy education and support to help students better manage their allergies, and allergy awareness information for the Queen’s University community at large.
Other steps taken to complement the new policy include making epinephrine available through emergency services and Campus Security, as well as an allergy education web site and an “Ask Us Before You Eat” awareness campaign near foodservice locations on campus.
The policy reflects many of Food Allergy Canada’s recommendations presented during their review process. Queen’s also engaged several other groups including students on campus, medical professionals, parents of students and other allergy experts.
Research
Ground-breaking new U.S. report promotes food allergy safety
Last November, the National Academies of Sciences, Engineering, and Medicine released a 562-page committee report titled “Finding a Path to Safety in Food Allergy”.
This report addresses the origins, prevalence, diagnosis, and management of food allergy in the U.S., and recommends improvements in research, training, advocacy, and public policy to better protect and support individuals affected by food allergy.
Some of the key recommendations in this comprehensive report include:
- Obtaining sound estimates of food allergy prevalence
- Implementing evidence-based diagnostic protocols and guidelines regarding food introduction
- Ensuring proper training in food allergy risk-reduction and anaphylaxis management for medical staff, patients with food allergies, caregivers, and foodservice personnel
- Making epinephrine available in public spaces such as schools, airplanes, and other facilities, and ensuring that staff in these locations are trained in anaphylaxis first response
- Labeling major allergens based on their prevalence, severity, and the potency of the allergens
- Ensuring that precautionary labelling is risk-based and standardized
U.S.-based FARE (Food Allergy Research & Education) was this project’s earliest advocate at its inception, as well as its lead sponsor. FARE supports the report’s call for the stronger protection of individuals with food allergies. To download the full report, please visit Finding a Path to Safety in Food Allergy.